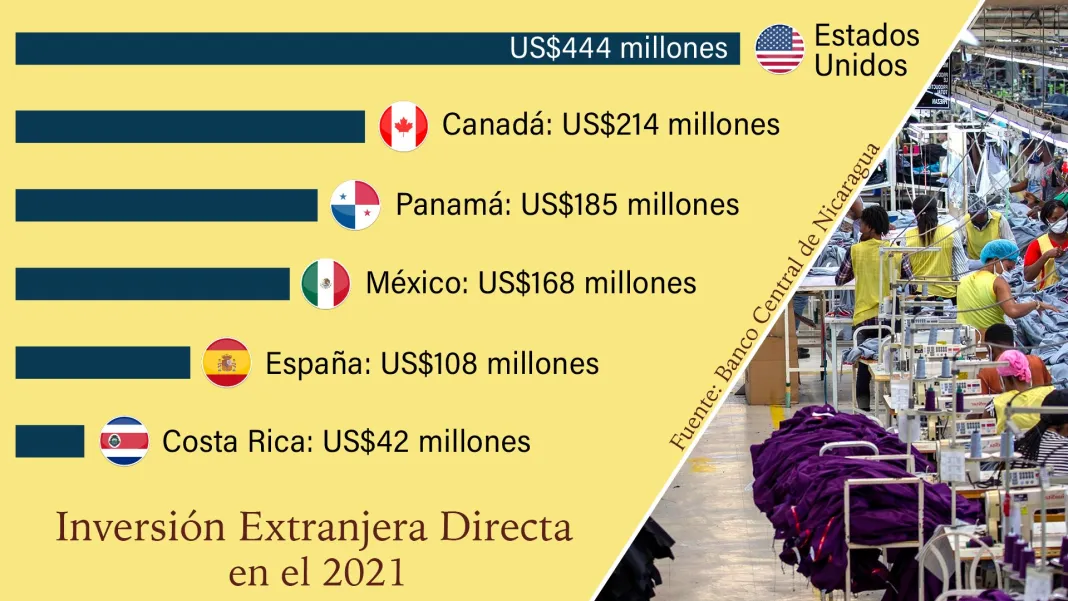
The Modern pharmaceutical company announced on Tuesday the start of phase 3 of its clinical study to develop an RNA-messenger vaccine against the respiratory syncytial virus (RSV) for people over 60 years of age, which will have 34,000 candidates in different countries, according to a company statement.
Moderna said the third phase is being launched “following an independent review of preliminary data from Phase 2, which suggests that the vaccine has an acceptable safety profile in older adults at the selected dose.”
The main objective of the third phase is to establish the safety and efficacy of the component in this population segment to support obtaining a license for its commercialization.
“RSV is one of the most widespread respiratory viruses, causing serious infections and hospitalization in older adults, and yet there is no vaccine available on the market,” Moderna CEO Stéphane Bancel said, quoted in the note.
According to Bancel, his vaccine could prevent more than a million infections globally each year.
In addition, RSV is the first cause of acute respiratory infection in children and is the main reason for lower respiratory tract hospitalization in children under two years of age in developed countries.
The drugmaker notes that while most people who get RSV recover in about a week or two, the virus can be serious for young children and older adults.
Moderna notes that in the United States, RSV causes about 177,000 hospitalizations and 14,000 deaths each year in adults 65 and older, resulting in an estimated annual medical cost of $3 billion.